Main content
Top content
Supramolecular Organogels
Gels are elastic materials consisting of a liquid solvent and at least one further component that forms a network interweaving the whole system. The solvent becomes physically attached to the interlacing structures and cannot leave the gel, though diffusive movements are still possible. Most gels are made up from macromolecules, either interconnected by chemical bonds (= solvent swollen crosslinked polymer) or via non-covalent interactions (thermoreversible gels).
It is known since long that several low molecular weight compounds (=”gelators”) are able to thermo-reversible gel water or organic liquids. Since a single small molecule is unable to span over large spatial dimensions, the gel-network must be formed by association of the gelator molecules into solid aggregates.
1. Amide Gelators
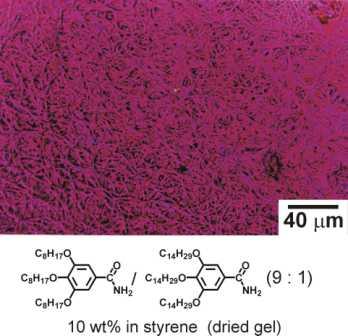
Figure 1.1: Optical micrograph of a dried organogel revealing the network of self-organised supramolecular fibre structures.
In fact, in some cases the gelator molecules self-assemble into long cylindrical superstructures (e.g. Figure 1.1) with perfect monodisperse diameters of molecular dimensions and well defined e.g. chiral superstructures. The rules governing the structure formation in the nanometer – regime of such ’supramolecular organogels’ are not well understood. Presently it is impossible to predict if an organic compound will form a gel together with a certain solvent, what type of structure will be formed or how fast the gelation process will proceed
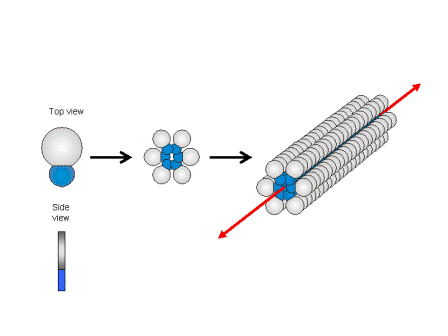
Figure 1.2a: Schematic sketch of the supramolecular fibre formation by wedge-shaped amphiphiles and some examples for this class of compounds.
Our research interest is to develop new low-molecular-weight gelators and to exploit the well defined supramolecular structures to generate functional materials, i.e. functional membranes or phase change materials. We follow a simple rational: The gel forming compounds consist of a large non-polar bulk attached to a small highly polar head group. In non-polar solvents the head groups try to avoid each contact to the non-polar enviroinment and associates with head-to-head arrangements of the molecules are favoured.
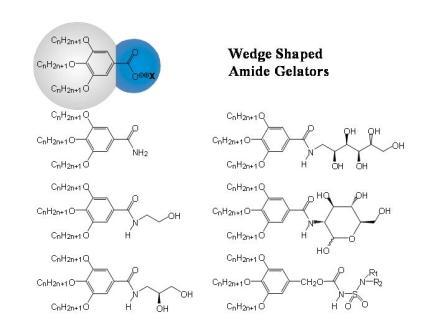
Figure 1.2b: Schematic sketch of the supramolecular fibre formation by wedge-shaped amphiphiles and some examples for this class of compounds.
Because of the package incomensurability between bulk and head, the formation of extended layers is suppressed and supramolecular structures with large curvatures at the molecule – solvent interface are obtained (cf. Figure 1.2)
The concept enables to synthesise a wide variety of gelation active structures. In addition the structures can be modified by proper combination of the solvent, the gelating temperatures and additives. Figure 1.3 depicts two examples for the supramolecular gel morphologies of N-deoxysorbityl-3,4,5-tris(dodecyloxy)benzamide in different methacrylate solvents. The gels have been polymerised subsequently to the gelation step, embedding the aggregates permanently into a crosslinked polymer matrix. We extended the concept to prepare dumb-bell shaped amphiphiles to increase the thermal- and mechanical stability of the resulting gels.
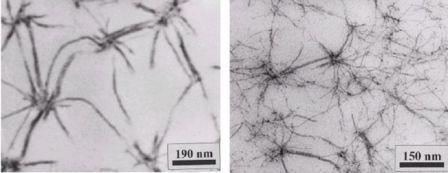
Figure 1.3: TEM-pictures of the gel-morphology of N-sorbitoyl-3,4,5-tris(dodecyloxy)benzamide (3 wt%) in (a) poly(hexylmethacrylate-cross-butanedioldimethacrylate) and (b) in poly(butylmethacrylate-cross-decanedioldimethacrylate)
2. Semicarbazide Gelators
Bis[alkoxybenzoyl]semicarbazides of the general structure Y-CO-NH-NH-CO-NH-X-NH-CO-NH-NH-CO-Y (Scheme 2.1) have been prepared.
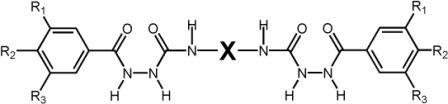
Scheme 2.1: General formula of the bis[(alkoxy)benzoyl semicarbazides (Ri = H-, CnH2n+1O-, n = 8, 10, 12, 16, X = 1,4-phenyl-, 2,4-toluyl-, 2,6-toluyl-, 1,8-naphtyl-, 1,6-hexyl-)
Y represents alkoxyphenyl units, and X denotes either an aromatic or an aliphatic moiety. Bis(semicarbazide) cores have been selected because of (i) their ability to form up to 14 hydrogen bonds per molecule, (ii) their simple preparation and (iii) their large structural variability. The compounds with X = 1,4-phenylene were found to be highly efficient organogelators that gelled semi-polar, and non-polar organic liquids. In hexane, toluene, styrene, and 1,3,5-tri(isopropyl)benzene (TiPrB) the critical gelation concentration was below 0.7 wt% (< 6 mmol/L, R = C8H17).
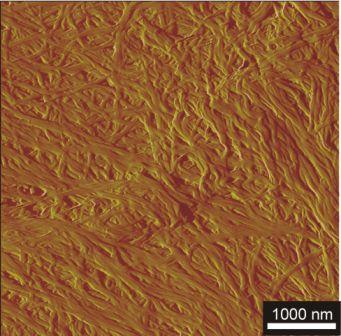
Figure 2.1: Morphology of a dried gel from 0.8 wt 3CBS-10 in THF : petrol ether (2:1)
Figure 2.1 depicts the morphology of a dried gel from the bis(semicarbazide) with X = 1,4-phenylene, and R = C10H25 (3CBS-10), obtained from a 0.8 wt% gel in THF : petrol ether (2 : 1). The gelator formed fibres of ? 100 nm diameter, and virtually infinite length. Bis(semicarbazides) offer the opportunity of ‘cold gelation’, i.e. ambient temperature gelation of low polarity liquids by mixing with highly concentrated solutions of a semicarbazide in e.g. DMAc. Gels arising from 3CBS-8 (X = 1,4-phenylene, R = C8H17) and TiPrB showed shear thickening effects on exceeding concentrations of 0.2 wt% (2.1 mmol/L), see Figure 2.2.
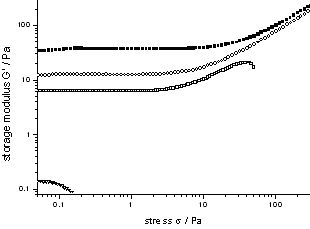
Figure 2.2: Storage modulus of gels from 3CBS-8 in TiPrB (0.75 wt%, 0.5 wt%, 0.25 wt%, 0.12 wt%) (Sigma = 0.1 Pa, Omega = 1 rad/s, T = 25 °C)
3. Sulfonate Gelators
Actual research is directed towards cunitic (i.e. wedge-shaped) gelator molecules that bear sulfonate groups at the tip of the wedge (c. Figure 3.1).
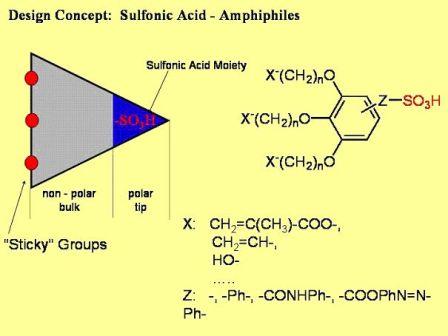
Figure 3.1: Cunitic sulfonate gelators presently under investigation

